FASTRAK Study
Children who are hard of hearing often wear hearing aids to help them hear and understand speech. Children who are hard of hearing and do not wear hearing aids are at risk of developing language delays, resulting from not hearing or understanding clearly.
It is difficult for audiologists to know which children may be at-risk for language delays because current clinical tools may not capture difficulties in hearing and understanding speech, especially in children with mild hearing levels. In addition, there are no clear clinical guidelines for audiologists to follow to know when hearing aids would be helpful for a child with mild hearing levels.
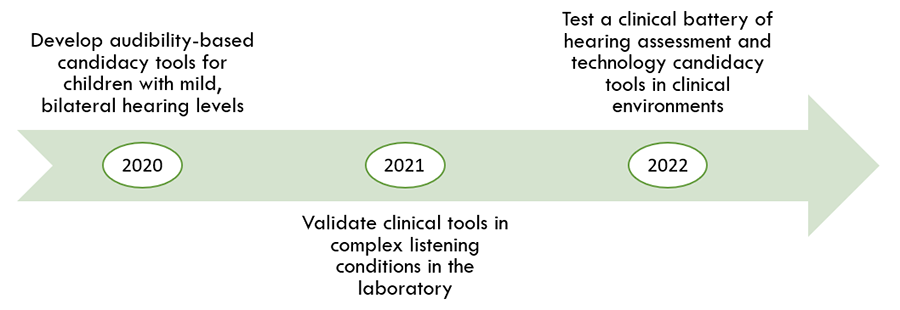
Our goal is to help audiologists determine when a child may benefit from hearing aids. To do that, we plan to develop clinical tools that can tell us when a child is experiencing difficulty and could benefit from a hearing aid. These tools will be very useful for audiologists who evaluate and treat children with mild hearing levels. The three stages of the study are shown below.
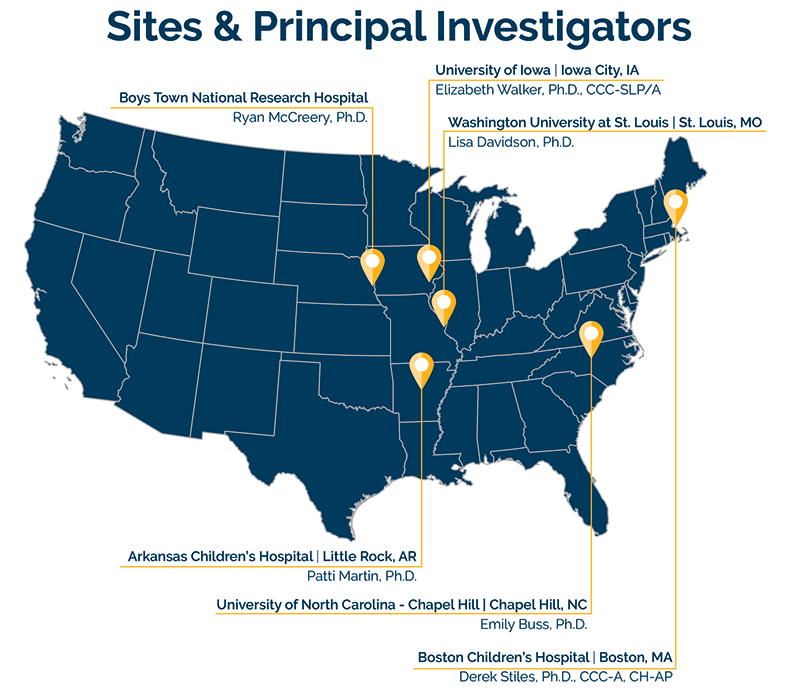
We will be partnering with clinical sites at Boston Children's Hospital, Arkansas Children's Hospital, University of Iowa, and Washington University at St. Louis.
Sites & Principal Investigators
Who Can Participate?
We are currently seeking children ages 4-12 years old with typical or mild hearing levels in both ears. Wearing hearing aids is not required. Children should be considered typically-developing and speak English as their native language. We are currently recruiting for the experimental phase of the FASTRAK study, which includes the sites at Boys Town National Research Hospital and the University of Iowa. The clinical phase of the FASTRAK study will begin at all sites, including Arkansas Children’s Hospital, Boston Children’s Hospital, and Washington University at St. Louis, in 2023.
Sign Up to Participate
To sign up to participate or for more information, please contact the site closest to you:
- Boys Town National Research Hospital (Omaha, NE):
- University of Iowa (Iowa City, IA):
Funding Information
Funding for FASTRAK is provided by the
NIH-NIDCD R01DC018330.
