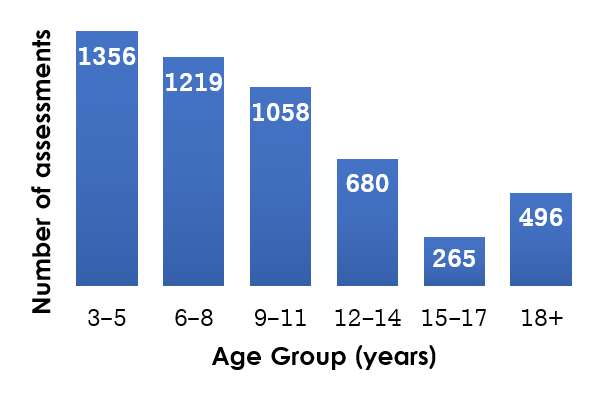Clinical Measurement Program (CMP)
The CMP team is comprised of a highly trained team of audiologists and speech-language pathologists with extensive experience supporting research projects.
Enhanced Measurement Quality
The CMP provides a variety of services to ensure that clinical measures used in research protocols are implemented with high levels of fidelity, thus resulting in the most accurate data possible. These services include consulting with PIs on selection, use and data analysis pertaining to clinical measures, administration and interpretation of clinical measures by the CMP (remotely or in person), and support for lab staff in labs administering their own clinical measures. The CMP can support measurement in a variety of domains, including speech, language, cognition, hearing and vision. To ensure that all support offered is of the highest quality, the CMP staff are all nationally certified clinicians with years of experience administering clinical measures in both clinical and research settings. For assessments outside of the CMP staff members' areas of expertise and/or clinical scope of practice, consultants are utilized to support CMP staff in selecting appropriate measures, ensure CMP staff are prepared to administer the measures with high fidelity and interpret assessment results.
The CMP's support of previous projects has included administering over 30 different assessment measures, ranging from language samples and vision screenings to standardized tests of speech, language and cognition. To date, the CMP has conducted assessments with over 700 participants, spanning in age from preschoolers through adults. Participants seen by the CMP include children and adults who are typically developing as well as those with hearing loss, developmental language disorders and Down syndrome.
Support for Protocol Development and Interpretation of Data
PIs are often faced with a need to measure clinical domains outside their direct area of expertise. In these situations, PIs may have limited practical experience with currently available clinical measures. The CMP staff are available in these situations to support PIs in selecting clinical measures that are well suited to answer the PIs question of interest, with an emphasis on choosing measures with strong psychometric properties. Similarly, the CMP staff can support PIs during data analysis with interpretation of results from individual clinical measures.
Quality Assurance Procedures for Measurement Completed by CMP Staff
When the CMP staff administers assessments, the team uses several procedures to ensure the data they collect is of the highest quality. First, the CMP regularly uses fidelity checklists developed by the team to monitor and provide feedback to team members on their implementation of clinical measures during participant visits. Second, all tests administered by the CMP are scored by two separate team members and consensus scoring is used to reach a decision on scoring discrepancies. Finally, all clinical assessment scores collected by the CMP team are double entered into databases to reduce potential for data entry errors.
Support for Labs Conducting Clinical Measures
When PIs have their own staff administer clinical measures, the CMP offers a variety of supports to ensure lab staff use the highest standards in administering and scoring the measures. PIs can request general training for their staff on conducting subject visits with a given population or training regarding a specific clinical measure or set of clinical measures. For specific clinical measures, a CMP team member meets with the appropriate lab staff, discusses the assessment and its procedures and shares the CMP fidelity checklist with the staff. The lab staff then each practice the assessment and, when comfortable, record a pilot subject. The CMP staff member uses the fidelity checklist to ensure the staff administer the assessment following standardized procedures. If fidelity can be improved, additional pilot subjects can be seen and further fidelity checks can be completed. Additionally, when requested by the PI, the CMP can double score each assessment completed by the lab staff or a percentage of the assessments for reliability reporting. In this process, a member of the PI's lab is the initial scorer and a CMP staff member is the second scorer.
Efficiency of Data Collection and Data Sharing
Efforts to increase the efficiency of data collection and data sharing have led to creation of the CMP Database. When the CMP administers and scores assessments for a project, the data are entered into REDCap and from there are accessible to lab staff on the protocol for the project. Additionally, assuming appropriate permissions were provided by the participant during consenting, the data are transferred to the CMP Database where they are available for secondary analysis and select data are transferred to the Recruitment Database maintained by the RQP. The CMP Database includes intake data and clinical measures scores for all participants seen by the CMP since its inception in 2014. See infographic for more information.